Simix Cleaner is photocatalytic
In today's world, where pollution and indoor air contaminants have become a growing concern, innovations in air purification technology are crucial. Among these innovations, Simix Cleaner with Nano Titanium Dioxide stands out as a remarkable solution. In this article, we'll delve into the science and benefits behind Simix Cleaner, explaining its ability to clean the air through photocatalytic activity in terms anyone can understand.
Clean air is essential for a healthy life, but it's not always readily available. Indoor pollutants like allergens, bacteria, and even volatile organic compounds (VOCs) can pose significant health risks. Simix Cleaner offers an innovative solution to this problem through the magic of nanotechnology and photocatalytic activity.
Simix Cleaner is a cutting-edge air purification cleaner designed to improve indoor air quality. It harnesses the power of nano titanium dioxide to efficiently remove impurities from the air you breathe. But what makes nano titanium dioxide so special?

The Power of Nano Titanium Dioxide
Tile Tales
Nano titanium dioxide, or TiO2, is a remarkable substance that, when exposed to light, becomes a powerful catalyst. It has the ability to break down organic and inorganic substances on a molecular level. This property is one of the keys to Simix Cleaner's effectiveness.
During the late 1970s, scientists began to realize that the propensity of TiO2 to absorb energy from the UV end of the solar spectrum and then react with water vapor to produce oxygen could be used to create surfaces that were, for all practical purposes, self-cleaning.
According to Daniel Blake, principal scientist at the Department of Energy’s National Renewable Energy Laboratory in Golden, Colorado, when TiO2 is exposed to UV light of a wavelength below 385 nanometers in the presence of water vapor, two highly reactive substances are formed: hydroxyl radicals [OH] and a superoxide ion [O2 -1]. Says Blake, “These are highly reactive chemical species. Hydroxyl radicals are very strong oxidizers and will attack all kinds of organic materials, including those that make up living cells.” Toto Frontier USA, based in Long Beach, California, is using TiO2 to make self-cleaning ceramic tiles for use in hospitals, public restrooms, and other settings where cleanliness is vitally important,
Simix Cleaner utilizes photocatalytic activity, a process that begins when nano titanium dioxide is exposed to light, typically from UV sources. When light interacts with the TiO2-coated surfaces , a chemical reaction occurs.
This reaction decomposes harmful airborne particles, transforming them into harmless substances like water vapor and carbon dioxide.
- Energy Efficiency: This innovative system consumes minimal energy while providing maximum air purification, making it environmentally friendly and cost-effective.
- Low Maintenance: Simix Cleaner requires no maintenance, just use it to clean everything in your restaurant and it will leave behind nano particles(that you cannot see) that will continue to work.
- Health Benefits: Cleaner air contributes to better respiratory health and reduces the risk of allergies and respiratory ailments.
Simix Cleaner's versatility allows it to be used in various settings, including homes, offices, schools, hospitals, and industrial facilities. It's a game-changer in any environment where air quality is a concern.
Improved Air Quality
Simix Cleaner effectively removes pollutants, allergens, and odors from the air, resulting in cleaner and fresher indoor air.
Simix Cleaner starts improving air quality almost immediately,especially in bathrooms, but noticeable differences may take a few hours to a day, depending on the room size and air quality.
Energy Efficiency
This innovative technology consumes minimal energy while providing maximum air purification, making it environmentally friendly and cost-effective.
As long as there is light available,it is working.
Is simix safe around pets and children?
Yes, Simix Cleaner is safe for both pets and children. It doesn't emit harmful byproducts and effectively removes allergens and pet odors.
How about smoke and cooking odors?
Absolutely. Simix Cleaner excels at removing smoke and cooking odors, leaving your indoor space fresh and clean.
Simix Cleaner can be used in industrial settings to improve air quality and reduce odors and contaminants.
Simix Cleaner is eco-friendly due to its energy efficiency and low maintenance requirements.
Simix Cleaner with Nano Titanium Dioxide is a revolutionary air purification system that uses photocatalytic activity to cleanse the air we breathe. Its ability to remove pollutants, improve air quality, and provide health benefits makes it an essential addition to any indoor environment. Say goodbye to indoor air concerns and hello to fresh, clean air with Simix Cleaner.
Simix also makes a ceramic coating that you can apply to your HVAC to further improve air quality
This cleaner has several intriguing properties. First, it can break down organic matter into carbon dioxide (CO2) and water. When organic matter lands on a TiO2-coated tile and water is applied, the hydroxyl radicals break down the cell wall and outer membrane, allowing cell contents to leak out and TiO2 particles to enter, thereby causing cell damage and death.
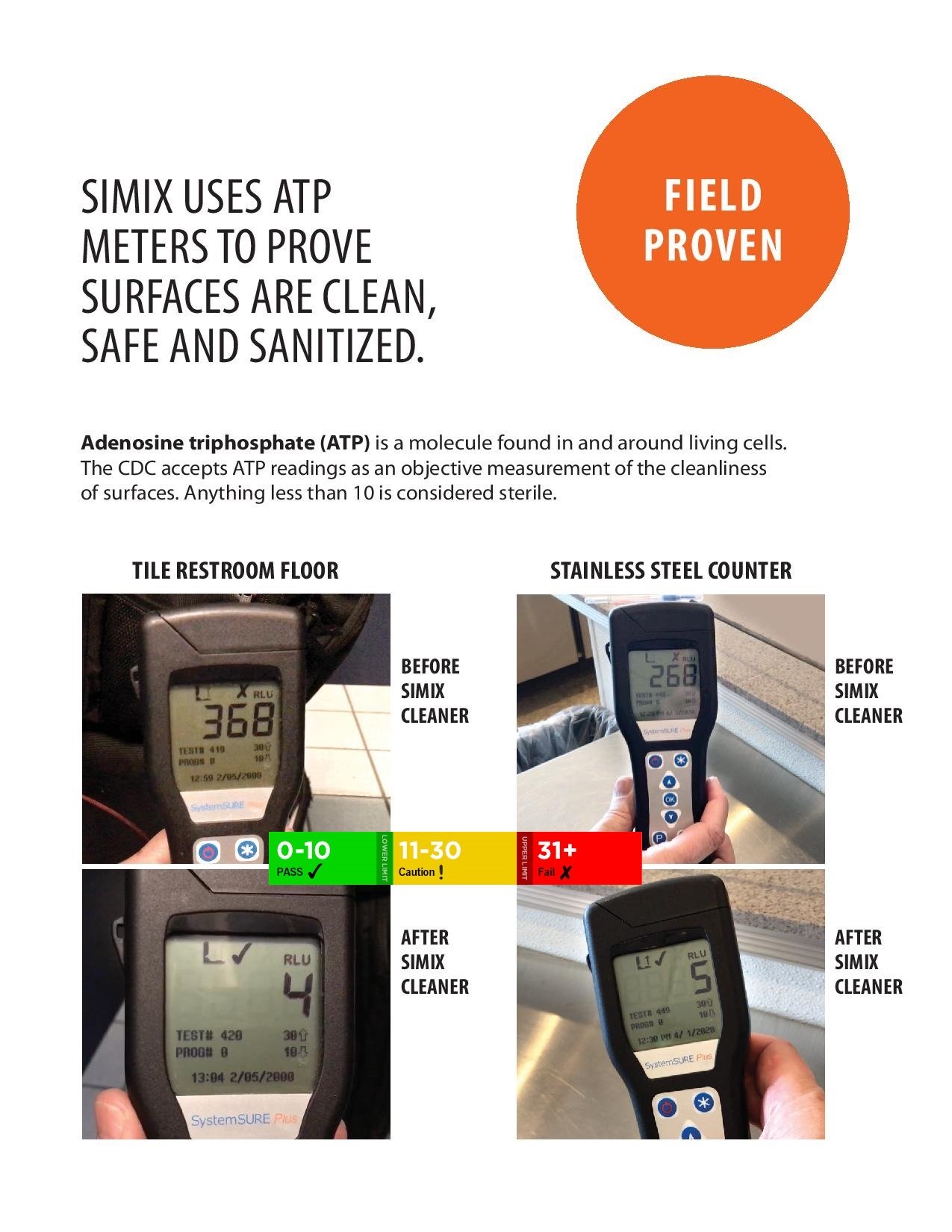 Anything under 10 is considered operating room sterile by the cdc
Anything under 10 is considered operating room sterile by the cdcHydroxyl radicals are highly reactive and thus short-lived. Superoxide ions, while longer-lived, cannot penetrate the cell membrane because of their negative charge. Therefore, both must interact immediately with the outer surface of an organism unless the TiO2 particle has already penetrated the cell. Research at the National Renewable Energy Laboratory shows that the process works equally well on bacterial and fungal spores. Tests performed and presented at a July 2000 Air and Waste Management Association symposium indicate that the TiO2-treated tiles achieve a 99.9% bacterial kill rate within one hour for such strains as penicillin-resistant Staphylococcus aureus and Escherichia coli. Another useful property of TiO2 is that it is superhydrophilic—the surface of a tile treated with it attracts water rather than repelling it. In the September 1999 issue of Ceramic Industry, Malloy explained the importance of this property: “
Watch Video to understand
These tiles are significantly easier to maintain in interior applications because they don’t require the use of
bleaches and detergents, wrote Malloy, and
they allow for the creation of exterior ceramic facades and roofs that are self-cleaning
with rainwater.
These surfaces are in fairly wide use throughout Japan and are being marketed in Europe as well, but they have not yet reached the U.S. market. Malloy says Toto is working on two products for the U.S. market including a self-cleaning exterior facade and interior tile surfaces. However, he says, there are regulatory roadblocks to cross first, especially for interior tile applications. “If we make the claim that the tile is antibacterial, we could be going outside of what is allowable under current Environmental Protection Agency standards,” he says.
Current standards
require a specific kill level within a certain
amount of time. TiO2 achieves the kill level
but in a longer period of time. However,
while slower than an agent like alcohol or
bleach, TiO2 is also less harmful to the environment, so it would be useful where a high
kill rate needs to be maintained over a long
period of time.
Road Warrior
Some of the 400 miles of pavement in the
Westminster borough of London may soon
have a role that goes far beyond smoothing
footpaths and roadways. Japan’s Mitsubishi
Materials Corporation has developed a
paving stone it calls “Noxer” that uses the
catalytic properties of TiO2 to remove nitrogen oxide (NOx
) from the air, breaking it
down into more environmentally benign
substances that can then be washed away by
rainwater. NOx is produced in abundance
through vehicle emissions.
The initial Noxer block design involved
a 200 millimeter (mm) by 100 mm block
with a 5–7 mm thick surface layer containing TiO2 mounted on a cement mortar base
to a total thickness of 60 mm. In tests by
Mitsubishi, the block was set in a reaction
vessel and exposed to a mixture of air and 1
part per million (ppm) NOx to simulate
vehicle emissions. A hole in the box allowed
for the addition of UV light, and a variety of
humidities were tested. According to
Mitsubishi, when the surface of the Noxer
slab is irradiated, oxygen is created, which
oxidizes NOx in the air into nitric acid ions.
These ions can then be washed away by
rainfall or neutralized by the alkaline composition of the concrete.
Results of these tests showed an 80%
NOx removal rate based on an intensity of
UV light of 1–12 watts per square meter
(W/m2) (the UV intensity of direct sunlight in summer is 20–30 W/m2, compared
to 1 W/m2 on a cloudy winter day). The
blocks functioned best under humidity conditions of 10–80%, with an inverse relationship between NOx removal and humidity.
According to Mitsubishi spokesperson
Yoshihiko Murata, in use outdoors the NOx removal rate varies depending on the concentration of NOx in the air. The company is currently conducting tests using computer simulations of pollution and actual street measurements. The Noxer blocks will be manufactured for testing by Marshalls (the United Kingdom’s largest manufacturer of landscape, building, and drainage materials for the construction industry and domestic home improvement markets) as a concrete base topped by a one-centimeter layer of a mixture of TiO2, zeolite, and sand, crushed quartz, or ground glass. Marshalls will use the same manufacturing processes and machinery as for their traditional products, but company PR manager Rachel Brown says, “In principle, there’s no reason why the Noxer technology could not be adapted for walling, tunnels, curbs, and so on to reduce the effects of vehicular emissions in the environment.” In addition to the proposed Westminster trials, Noxer blocks are also being tested in Osaka and Chiba, Japan. “NOx removal using TiO2 coatings and additions to paving materials and large-scale panels on road sides and the like is a very practical approach,” says Blake. “It’s passive, in the sense that it relies on natural sunlight and air movement, and no lamps or fans are required; thus, it’s simple to implement. Adverse effects of the system seem unlikely because the quantity of the NOx is very low to begin with, and it’s distributed over large areas.” Reynaldo Barreto, a chemistry professor at Purdue University in West Lafayette, Indiana, agrees it is an interesting idea, but, he says, “I think we’d be better off dealing with NOx as it comes out of the exhaust. NOx is a product of high-temperature combustion, and it’s something we have to deal with one way or another. If you tried to do it in a tunnel with the proper lighting spectrum it might work, but on the pavement? Seems like a hard way to make your point.” A Fresh Application In addition to cleansing the air outside, TiO2 is being used to treat the air in fruit, vegetable, and cut flower storage areas to prevent spoilage and increase the products’ shelf life. The Kennesaw, Georgia–based KES Science & Technology has commercialized the work of Marc Anderson, a professor of environmental engineering and materials science at the University of Wisconsin at Madison to create an enclosed system that uses the photocatalytic properties of TiO2 to remove ethylene from the air. Ethylene is a naturally occurring gaseous growth hormone produced by plant tissue that in concentrations as low as 1 ppm triggers the ripening of fruits and vegetables. Ethylene is also produced from other sources including internal combustion engines, certain fungi, and cigarette smoke. Originally developed for use aboard the space shuttle, the Bio-KES system breaks down ethylene into CO2 and water. According to Anderson, the University of Wisconsin was active in producing plant growth chambers for shuttle experiments. He says, “We had to develop a way to remove the ethylene from the air because otherwise it would retard growth and spoil the plants. Conventional filtration would just remove particulates from the air, but we needed a way to break down this gaseous substance in an efficient manner while producing no hazardous by-products.” The Bio-KES system has been used successfully on six shuttle missions since 1993. The system draws air through small borosilicate tubes (4 mm in diameter by 12 mm long) that have been lined with a TiO2 coating. Each system can handle volumes up to 10,000 cubic feet and contains 48 eight-watt UVA bulbs and six eightwatt UVC bulbs. According to KES Science & Technology, the combined action of UV light and TiO2 oxidizes ethylene into CO2 and water vapor at a rate of more than 32 milliliters of ethylene gas per hour. University of Wisconsin researchers are working on a treatment for the TiO2 crystals that would accelerate the reaction by four to six times. The Bio-KES system is currently being used primarily by commercial florists. KES Science & Technology is also developing models for use in sea/land cargo containers and in truck and train transportation. Diverse Decontaminator Still other researchers are working on ways to use TiO2’s properties to benefit the environment. Barreto has developed an experimental method of removing the gasoline additive methyl-tert-butyl ether (MTBE, used as an octane enhancer) from water by bubbling oxygen into the contaminated water and adding TiO2, a process that converts both MTBE and another additive, tert-butyl alcohol, to CO2 with a 90% efficiency rate in as little as four hours. MTBE is a potential human carcinogen, and concerns have been raised about its potential for acute effects from exposures by inhalation and long-term exposures from drinking water. Barreto’s technology is still under study and has not yet been commercially developed. Researchers at Robert Gordon University in Aberdeen, Scotland, have experimented with degrading microcystins (toxins produced by cyanobacteria that are linked with liver cancer and can cause death in immunosuppressed people) by simply stirring in TiO2 and exposing the water to UV light. Results show a complete degradation of the toxin microcystin-LR in less than 20 minutes. Says Blake, “[This application] is quite interesting because the TiO2 will work under ambient sunlight. This has the potential for applications where one could use solar ponds [artificially created, enclosed bodies of water that use ambient light as part of a treatment process] or other means to treat large areas or quantities of water where the processing rate didn’t have to be on the order of millions of gallons a day.” Research suggests that TiO2 may be useful in other applications to remove organic contaminants from water. In experiments at Sandia National Laboratories in which contaminated water was flowed through glass pipes lined with TiO2 while being exposed to UV light, researchers were able to reduce concentrations of trichloroethylene (TCE) from 1.2 ppm to less than 50 parts per billion in only 5 minutes. (The project never reached commercialization, however.) Michael Prairie, a principal investigator at Sandia, was lead investigator on a number of TiO2 research projects at Sandia in the early 1990s, including using TiO2 to remove explosives residue from water, work that he says was eventually A 176 VOLUME 109 | NUMBER 4 | April 2001 • Environmental Health Perspectives Innovations • Environmental White Knight? R
Road Warrior
Some of the 400 miles of pavement in the Westminster borough of London may soon have a role that goes far beyond smoothing footpaths and roadways. Japan’s Mitsubishi Materials Corporation has developed a paving stone it calls “Noxer” that uses the catalytic properties of TiO2 to remove nitrogen oxide (NOx ) from the air, breaking it down into more environmentally benign substances that can then be washed away by rainwater. NOx is produced in abundance through vehicle emissions. The initial Noxer block design involved a 200 millimeter (mm) by 100 mm block with a 5–7 mm thick surface layer containing TiO2 mounted on a cement mortar base to a total thickness of 60 mm. In tests by Mitsubishi, the block was set in a reaction vessel and exposed to a mixture of air and 1 part per million (ppm) NOx to simulate vehicle emissions. A hole in the box allowed for the addition of UV light, and a variety of humidities were tested. According to Mitsubishi, when the surface of the Noxer slab is irradiated, oxygen is created, which oxidizes NOx in the air into nitric acid ions. These ions can then be washed away by rainfall or neutralized by the alkaline composition of the concrete. Results of these tests showed an 80% NOx removal rate based on an intensity of UV light of 1–12 watts per square meter (W/m2) (the UV intensity of direct sunlight in summer is 20–30 W/m2, compared to 1 W/m2 on a cloudy winter day). The blocks functioned best under humidity conditions of 10–80%, with an inverse relationship between NOx removal and humidity. According to Mitsubishi spokesperson
Yoshihiko Murata, in use outdoors the NOx removal rate varies depending on the concentration of NOx in the air. The company is currently conducting tests using computer simulations of pollution and actual street measurements. The Noxer blocks will be manufactured for testing by Marshalls (the United Kingdom’s largest manufacturer of landscape, building, and drainage materials for the construction industry and domestic home improvement markets) as a concrete base topped by a one-centimeter layer of a mixture of TiO2, zeolite, and sand, crushed quartz, or ground glass. Marshalls will use the same manufacturing processes and machinery as for their traditional products, but company PR manager Rachel Brown says, “In principle, there’s no reason why the Noxer technology could not be adapted for walling, tunnels, curbs, and so on to reduce the effects of vehicular emissions in the environment.” In addition to the proposed Westminster trials, Noxer blocks are also being tested in Osaka and Chiba, Japan. “NOx removal using TiO2 coatings and additions to paving materials and large-scale panels on road sides and the like is a very practical approach,” says Blake. “It’s passive, in the sense that it relies on natural sunlight and air movement, and no lamps or fans are required; thus, it’s simple to implement. Adverse effects of the system seem unlikely because the quantity of the NOx is very low to begin with, and it’s distributed over large areas.” Reynaldo Barreto, a chemistry professor at Purdue University in West Lafayette, Indiana, agrees it is an interesting idea, but, he says, “I think we’d be better off dealing with NOx as it comes out of the exhaust. NOx is a product of high-temperature combustion, and it’s something we have to deal with one way or another. If you tried to do it in a tunnel with the proper lighting spectrum it might work, but on the pavement? Seems like a hard way to make your point.” A Fresh Application In addition to cleansing the air outside, R
TiO2 is being used to treat the air in fruit, vegetable, and cut flower storage areas to prevent spoilage and increase the products’ shelf life
The Kennesaw, Georgia–based KES Science & Technology has commercialized the work of Marc Anderson, a professor of environmental engineering and materials science at the University of Wisconsin at Madison to create an enclosed system that uses the photocatalytic properties of TiO2 to remove ethylene from the air. Ethylene is a naturally occurring gaseous growth hormone produced by plant tissue that in concentrations as low as 1 ppm triggers the ripening of fruits and vegetables. Ethylene is also produced from other sources including internal combustion engines, certain fungi, and cigarette smoke. Originally developed for use aboard the space shuttle, the Bio-KES system breaks down ethylene into CO2 and water. According to Anderson, the University of Wisconsin was active in producing plant growth chambers for shuttle experiments. He says, “We had to develop a way to remove the ethylene from the air because otherwise it would retard growth and spoil the plants. Conventional filtration would just remove particulates from the air, but we needed a way to break down this gaseous substance in an efficient manner while producing no hazardous by-products.” The Bio-KES system has been used successfully on six shuttle missions since 1993. The system draws air through small borosilicate tubes (4 mm in diameter by 12 mm long) that have been lined with a TiO2 coating. Each system can handle volumes up to 10,000 cubic feet and contains 48 eight-watt UVA bulbs and six eightwatt UVC bulbs. According to KES Science & Technology, the combined action of UV light and TiO2 oxidizes ethylene into CO2 and water vapor at a rate of more than 32 milliliters of ethylene gas per hour. University of Wisconsin researchers are working on a treatment for the TiO2 crystals that would accelerate the reaction by four to six times. The Bio-KES system is currently being used primarily by commercial florists. KES Science & Technology is also developing models for use in sea/land cargo containers and in truck and train transportation. Diverse Decontaminator Still other researchers are working on ways to use TiO2’s properties to benefit the environment. Barreto has developed an experimental method of removing the gasoline additive methyl-tert-butyl ether (MTBE, used as an octane enhancer) from water by bubbling oxygen into the contaminated water and adding TiO2, a process that converts both MTBE and another additive, tert-butyl alcohol, to CO2 with a 90% efficiency rate in as little as four hours. MTBE is a potential human carcinogen, and concerns have been raised about its potential for acute effects from exposures by inhalation and long-term exposures from drinking water. Barreto’s technology is still under study and has not yet been commercially developed. Researchers at Robert Gordon University in Aberdeen, Scotland, have experimented with degrading microcystins (toxins produced by cyanobacteria that are linked with liver cancer and can cause death in immunosuppressed people) by simply stirring in TiO2 and exposing the water to UV light. Results show a complete degradation of the toxin microcystin-LR in less than 20 minutes. Says Blake, “[This application] is quite interesting because the TiO2 will work under ambient sunlight. This has the potential for applications where one could use solar ponds [artificially created, enclosed bodies of water that use ambient light as part of a treatment process] or other means to treat large areas or quantities of water where the processing rate didn’t have to be on the order of millions of gallons a day.” Research suggests that TiO2 may be useful in other applications to remove organic contaminants from water. In experiments at Sandia National Laboratories in which contaminated water was flowed through glass pipes lined with TiO2 while being exposed to UV light, researchers were able to reduce concentrations of trichloroethylene (TCE) from 1.2 ppm to less than 50 parts per billion in only 5 minutes. (The project never reached commercialization, however.) Michael Prairie, a principal investigator at Sandia, was lead investigator on a number of TiO2 research projects at Sandia in the early 1990s, including using TiO2 to remove explosives residue from water, work that he says was eventually A 176 VOLUME 109 | NUMBER 4 | April 2001 • Environmental Health Perspectives Innovations • Environmental White Knight?




